Dissolution DNP
DNP is now a well-established technique that is facilitated by the transfer of the polarization of unpaired electrons to the nuclei under study. Dissolution DNP methods require the sample be kept cold (e.g., <4 K) while irradiated by microwaves in a strong magnetic field (e.g., 3 T). After irradiation, the sample is rapidly dissolved using hot pressurized solvent and transferred to 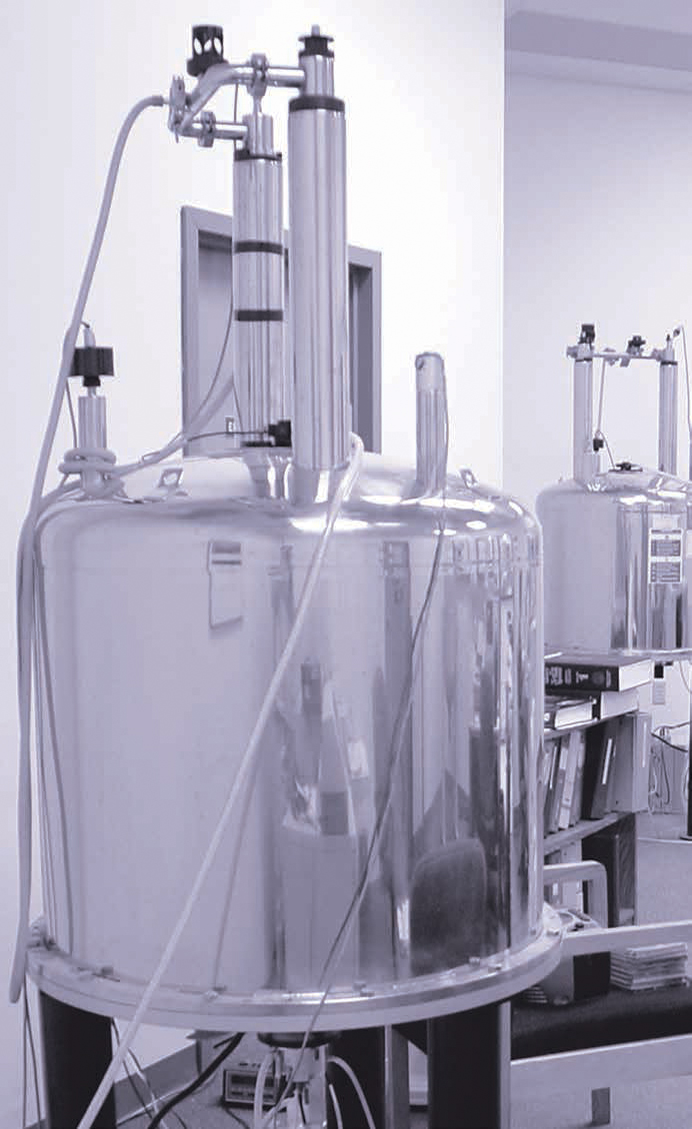 a NMR tube or an injection syringe for immediate NMR or MRI data acquisition. A free-radical doping agent must be present in the sample at mM concentrations during microwave irradiation in order to provide the needed nuclear polarisation for intermolecular transfer. Generally, acceptable levels of polarisation are achieved using irradiation times that are several tens of minutes to several hours in length.
a NMR tube or an injection syringe for immediate NMR or MRI data acquisition. A free-radical doping agent must be present in the sample at mM concentrations during microwave irradiation in order to provide the needed nuclear polarisation for intermolecular transfer. Generally, acceptable levels of polarisation are achieved using irradiation times that are several tens of minutes to several hours in length.
Low-temperature DNP, a method pioneered by the laboratory of Robert Griffin, however, is a slightly different technique than dissolution DNP because both hyperpolarisation and nmr acquisition take place in the solid state and where the hyperpolarised signal is effectively continually regenerated which allows for data acquisition from hours to many days.
Parahydrogen-Induced Polarization (PHIP)
PHIP is a fairly well-established technique that transfers polarization directly from parahydrogen (para-H2) to nearby nuclei of interest or by using RF-based magnetisation transfer methods. For organic molecules, para-H2 is either added directly across unsaturated carbon bonds or mixed with the sample under conditions such that polarisation can transfer from para-H2 to sites within the molecule. Some advantages of PHIP over DNP are that a polarised sample may be attained quite quickly (on the order of seconds or minutes) and that free-radical doping agents are not required.
Metabolic Imaging
Perhaps the most exciting consequence of signal enhancement obtained using DNP and PHIP is the potential for in vivo 1H,13C and 15N monitoring of metabolism. In particular, 13C MRI imaging using hyperpolarised 13C enriched organic molecules offers significant advantages over 1H-based imaging techniques because background signals are not detected and the large chemical shift range for 13C leads to increased molecular selectivity. Currently there is interest in the use of isotopically enriched, hyperpolarised substrates for medical imaging because detailed, metabolic information (substrate localisation and biochemical transformations) and physiological information (e.g., intracellular pH) may be obtained. Although the signal enhancement of hyperpolarised spin one-half nuclei decays with T1, research is underway to establish long-lived nuclear states with the promise that metabolism may be studied over time scales of minutes or even hours instead of seconds.
Please search our catalogue for any products of interest or contact us to discuss any element.
Listed below are our Structural Biomolecular NMR catalogue and a couple of application notes written by CIL customers.
Hyperpolarization/MRI/MRS
Product Search
Got a Question?
For information please start your enquiry below:



